Introduction
Textiles, yarns, and raw fibers need to be clean before dyeing and mordanting to allow the dye and mordant to bind the fiber. In the following paragraphs we will explain importance and fundamentals of these procedures.
Scouring
This is a process that uses detergents (surfactants) to clean the fibers. Contaminants like grease, dirt, and other chemicals added to the fabric as filler or glaze (sizing) need to be removed to allow for better interaction between the fiber and dye or mordant.
Raw animal fibers would contain grease, waxes, dirt, sweat, urine, and other contaminants that could reach up to 30% of the weight of the fiber. Plant fibers too may contain wax, spinning oil, starch, and other sizing chemicals. Because all dye baths and mordants are prepared in water, any greasy coating would prevent the dye and mordant from reaching the fiber and an uneven dyeing would result (water and oil do not mix).
Different fibers require different scouring processes. Neutral detergents are used, for example Orvus1 and Synthropol.2 The acidity or alkalinity (pH) of the detergent is important because it can wash out the dye, additionally alkaline soaps and detergents can damage the fiber, importantly if they are protein fibers like wool and silk.
The chemistry of surfactants
Detergents are surfactants. The word surfactant comes from surface-active-reagent. They perform as detergents and wetting reagents due to their property of reducing the surface tension of water, this means that they allow water molecules to bind other surfaces by reducing the attraction among themselves. Surfactants are made of a long non-polar tail (hydrophobic = water fear) and a polar head (hydrophilic = water love) (Fig. 1).
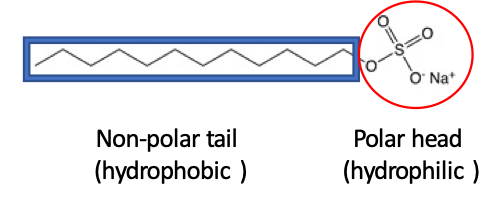
The non-polar tails have the same properties as fats and oils and because “like dissolves like”, they readily trap dirt. The polar heads remain in contact with the water forming a spheric particle called a micelle. The micelles remain suspended in water and are removed during the rinse. It will be useful that you remember that fatty compounds will interact more strongly with other fatty compounds than water (Fig. 2).
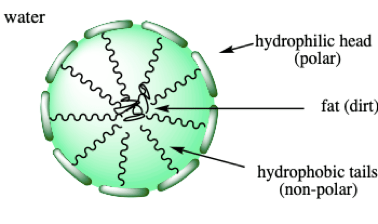
Why not using common detergents?
Common detergents are not a single chemical but rather a mixture of chemicals as listed below:
- Optical brighteners: these are fluorescent washfast dyes that make fabric look brighter.
- Softeners which stick to the fabric and may attract dirt.
- Enzymes added to remove tough organic stains.
- Fragrances
Orvus
This is the brand name of sodium lauryl sulfate (SLS),3 a detergent, Fig. 1. It is a petroleum-based chemical which is used as “horse shampoo” and “quilt shampoo”.
Sodium lauryl sulfate is a common ingredient in cosmetic products, although it is formulated with co-surfactants (other milder detergents) to reduce its skin irritant effects.3
Safety
Use gloves and mask to manipulate SLS; flakes and powder can easily disperse in the air. It may irritate the skin and cause allergic reactions. SLS is considered a sustainable material because it is biodegradable and has low potential for bioaccumulation.
Synthrapol
This product is a proprietary formulation that contains water, propanol (rubbing alcohol), and ethoxylated and sulphated aliphatic alcohols (non-ionic surfactants).
Synthrapol is useful to remove sizing before dyeing and excess (acid) dyes as they are soluble in the alcohol. It is also a wetting reagent and has neutral pH.
The detergent in the formulation eliminates the excess dye by the same mechanism that it removes dirt, that is by forming micelles (Fig. 3). The alcohol allows for a better solubilization of the excess dye in water and it prevents its reattachment to the fabric.
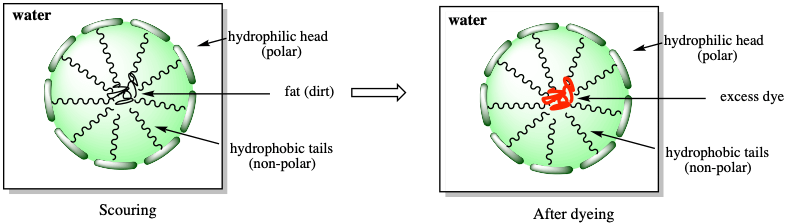
Why not using common soap or other detergents?
Soaps are like detergents because they have a hydrophobic tail, but the polar head as well as the manufacturing process is different. Soaps have a carboxylate group (-COO–) as the hydrophilic head which can form solids in water containing calcium, magnesium, and iron (hard waters), this solid attach to the fabric. Soaps have high pH (alkaline) because they are manufactured with sodium hydroxide (NaOH, lye). Soaps may not dissolve excess dyes efficiently -what the alcohol in Synthrapol does-, they can also damage protein fibers.
Safety
Synthrapol may cause irritation to the eyes, skin, and upper respiratory track. It is a combustible liquid. Small quantities can be disposed in the waste waters.
Mordanting
Mordanting is the most important process of preparing fibers to bind the dyes as they increase the affinity for the dye.4 In the chemical sense, mordants form coordination complexes with the fiber and with the dye (Fig. 4). Mordants are usually inorganic type of compounds containing a heavy metal. The metal increases the binding and the fastness of dyes. It is also responsible of providing a range of colors and hues.
Metals in common mordants are aluminum (Al), copper (Cu), iron (Fe), and tin (Sn) ions.
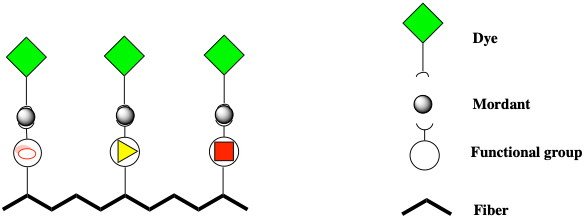
The weight of fabric (W.O.F.) needs to be determined to calculate the weight of mordant needed. This procedural step can be rationalized based on the average number of reactive “sites” (functional groups) present in each type of fabric. For example, the amount of ammonium groups in wool is estimated to be about 850 mmol/kg, in silk 250 mmol/kg and in polyamide 30– 50 mmol/kg. Even if you are not familiar with the units (mmol = millimole), the different number of reactive sites in each fiber determines the approximate amount of mordant that could be absorbed per W.O.F.
Mordants are applied before the dye and not in conjunction. When mordants are mixed with soluble dye they react forming a very fine powder (lake) that is insoluble in water; this reaction reduces the concentration of reagents available for the fabric.
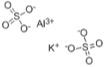
A common mordant is potassium aluminum sulfate dodecahydrate or alum, [KAl(SO4)2.12 H2O] (Fig. 5, anhydrous form); it is inexpensive and it is used to mordant protein fibers. When reacted with sodium carbonate (Na2CO3) also known as soda ash, it becomes a good mordant for cellulose fibers.
Tannins and tannic acid are non-metallic natural products and are also used as mordants4 (Fig. 6). Tannins and tannic acid are products derive from plants, such as tea leaves and tree bark. They are useful to fix basic dyes because they are acidic, and they can react readily forming a strong bond.
Safety
Prolonged exposure to heavy metals in the mordants may cause health problems such as kidney failure, emphysema, allergies, and even cancer. The maximum content of certain metals in the final product are regulated.5 The textile industry applies for certifications such as Aeko-Tex6 and Global Organic Textile Standard (GOT),7 to inform that their products comply with the standards and that are safe. Some metals with maximum limit are arsenic (As), lead (Pb), cobalt (Co), nickel (Ni), chromium (Cr), and zinc (Zn). Metallic mordant containing aluminum, iron, copper, and tin do not have a maximum limit, but reasonable use should be exercised to avoid environmental pollution.
Cited Literature
1 https://www.generations-quilt-patterns.com/orvus.html
2 https://sewingiscool.com/synthrapol-substitutes-ingredients/
3 Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE. Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environ Health Insights. 2015 (9) 27-32. doi: 10.4137/EHI.S31765.
4 Ellis C and Boutrup J. The Art and Science of Natural Dyes: Principles, Experiments, and Results. Schiffer Publishing, Ltd., Atglen, PA, 2018.
5 https://www.hindawi.com/journals/jspec/2015/640271/
6 https://www.parachutehome.com/blog/oeko-tex-textile-certification
7 https://global-standard.org/
